Hair Cell Damage and Repair
Hearing loss is America’s leading disability, affecting millions of people of all ages. To develop preventative and restorative clinical approaches, it is crucial to understand how the hearing process works on the cellular and molecular level. Hearing is mediated by sensory hair cells, part of a highly specialized neuroepithelium in the inner ear. Given that mammalian hair cells do not regenerate, the repair of hair cell damage is important for continued auditory function throughout life. Our lab therefore focuses on the molecules and mechanisms involved in hair cell repair and maintenance.
Hearing loss is America’s leading disability, affecting millions of people of all ages. To develop preventative and restorative clinical approaches, it is crucial to understand how the hearing process works on the cellular and molecular level. Hearing is mediated by sensory hair cells, part of a highly specialized neuroepithelium in the inner ear. Given that mammalian hair cells do not regenerate, the repair of hair cell damage is important for continued auditory function throughout life. Our lab therefore focuses on the molecules and mechanisms involved in hair cell repair and maintenance.
Hearing loss is America’s leading disability, affecting millions of people of all ages. To develop preventative and restorative clinical approaches, it is crucial to understand how the hearing process works on the cellular and molecular level. Hearing is mediated by sensory hair cells, part of a highly specialized neuroepithelium in the inner ear. Given that mammalian hair cells do not regenerate, the repair of hair cell damage is important for continued auditory function throughout life. Our lab therefore focuses on the molecules and mechanisms involved in hair cell repair and maintenance.
Our research interests can be divided into three categories:
1. Repair and maintenance of hair cell function and structure
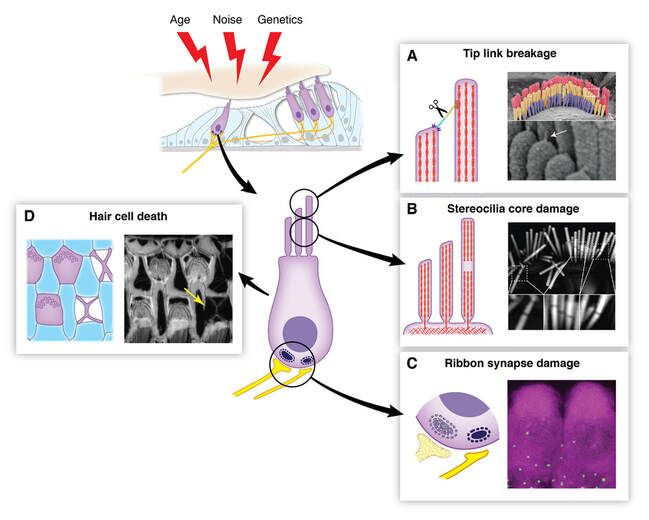
Sensory hair cells of the inner ear are exposed to continuous mechanical stress, causing damage over time. The maintenance of hair cells is further challenged by damage from a variety of other ototoxic factors, including loud noise, aging, genetic defects, and ototoxic drugs. This damage can manifest in many forms, from dysfunction of the hair cell mechanotransduction complex to hair cell death. Because mammalian hair cells do not regenerate, the repair of hair cell damage is important for continued auditory function throughout life. The majority of my research program is devoted to the question of how the hair cell repairs itself.
I) Repair of the stereocilia actin core: We hypothesize that a protein called XIRP2 is essential for the repair of the actin cytoskeletal structures that make up the mechanosensory hair bundle.
II) Cuticular plate: A more recent interest concerns molecules (e.g. LMO7) that are indispensable for the integrity of the cuticular plate, which provides the mechanical foundation for the hair bundle.
III) Repair of the hair cell mechanotransduction complex: A long-standing interest of the lab has been the repair of the tip link and mechanotransduction (MET) complex. Our most recent work has established that Myosin-VIIa is the tip link motor that provides tension to the MET complex and is essential for the sensitivity of hearing. Our next step is to study the mechanisms that mediate the repair of the tip link and MET complex, which can be damaged by external stresses such as age and noise.
2. How do ototoxic drugs kill hair cells, and how can we prevent it?
Aminoglycosides and cisplatin comprise a potent class of antibiotics and anticancer drugs, respectively, but their clinical use is limited due to ototoxicity. Despite longstanding research efforts, our understanding of the mechanisms underlying aminoglycoside and cisplatin ototoxicity is insufficient, and methods for clinical intervention have yet to emerge. We have recently found that the regulation of protein homeostasis in hair cells is severely affected by aminoglycosides and cisplatin. Protein homeostasis is at the center of general cellular homeostasis, and its dysregulation can activate various stress pathways leading to cellular degeneration and death. In addition, we are exploring the involvement of novel stress and cell death pathways (such as necroptosis) in aminoglycoside- and cisplatin-induced hair cell death, with special emphasis on the discovery of novel drugs to prevent hair cell degeneration by blocking stress pathways.
3. Discovery of novel proteins involved in hearing and deafness
This is our discovery pipeline, by which we identify, in an unbiased manner, proteins that are involved in hair cell function. We identify novel hair bundle proteins using a proteomics approach, characterize the function of these proteins, and generate mouse models to evaluate their role in hearing in vivo. Presently, we are applying this workflow on 10+ different potential deafness genes.
Our research interests can be divided into three categories:
1. Repair and maintenance of hair cell function and structure
Sensory hair cells of the inner ear are exposed to continuous mechanical stress, causing damage over time. The maintenance of hair cells is further challenged by damage from a variety of other ototoxic factors, including loud noise, aging, genetic defects, and ototoxic drugs. This damage can manifest in many forms, from dysfunction of the hair cell mechanotransduction complex to hair cell death. Because mammalian hair cells do not regenerate, the repair of hair cell damage is important for continued auditory function throughout life. The majority of my research program is devoted to the question of how the hair cell repairs itself.
I) Repair of the stereocilia actin core: We hypothesize that a protein called XIRP2 is essential for the repair of the actin cytoskeletal structures that make up the mechanosensory hair bundle.
II) Cuticular plate: A more recent interest concerns molecules (e.g. LMO7) that are indispensable for the integrity of the cuticular plate, which provides the mechanical foundation for the hair bundle.
III) Repair of the hair cell mechanotransduction complex: A long-standing interest of the lab has been the repair of the tip link and mechanotransduction (MET) complex. Our most recent work has established that Myosin-VIIa is the tip link motor that provides tension to the MET complex and is essential for the sensitivity of hearing. Our next step is to study the mechanisms that mediate the repair of the tip link and MET complex, which can be damaged by external stresses such as age and noise.
2. How do ototoxic drugs kill hair cells, and how can we prevent it?
Aminoglycosides and cisplatin comprise a potent class of antibiotics and anticancer drugs, respectively, but their clinical use is limited due to ototoxicity. Despite longstanding research efforts, our understanding of the mechanisms underlying aminoglycoside and cisplatin ototoxicity is insufficient, and methods for clinical intervention have yet to emerge. We have recently found that the regulation of protein homeostasis in hair cells is severely affected by aminoglycosides and cisplatin. Protein homeostasis is at the center of general cellular homeostasis, and its dysregulation can activate various stress pathways leading to cellular degeneration and death. In addition, we are exploring the involvement of novel stress and cell death pathways (such as necroptosis) in aminoglycoside- and cisplatin-induced hair cell death, with special emphasis on the discovery of novel drugs to prevent hair cell degeneration by blocking stress pathways.
3. Discovery of novel proteins involved in hearing and deafness
This is our discovery pipeline, by which we identify, in an unbiased manner, proteins that are involved in hair cell function. We identify novel hair bundle proteins using a proteomics approach, characterize the function of these proteins, and generate mouse models to evaluate their role in hearing in vivo. Presently, we are applying this workflow on 10+ different potential deafness genes.